Improve therapy selection
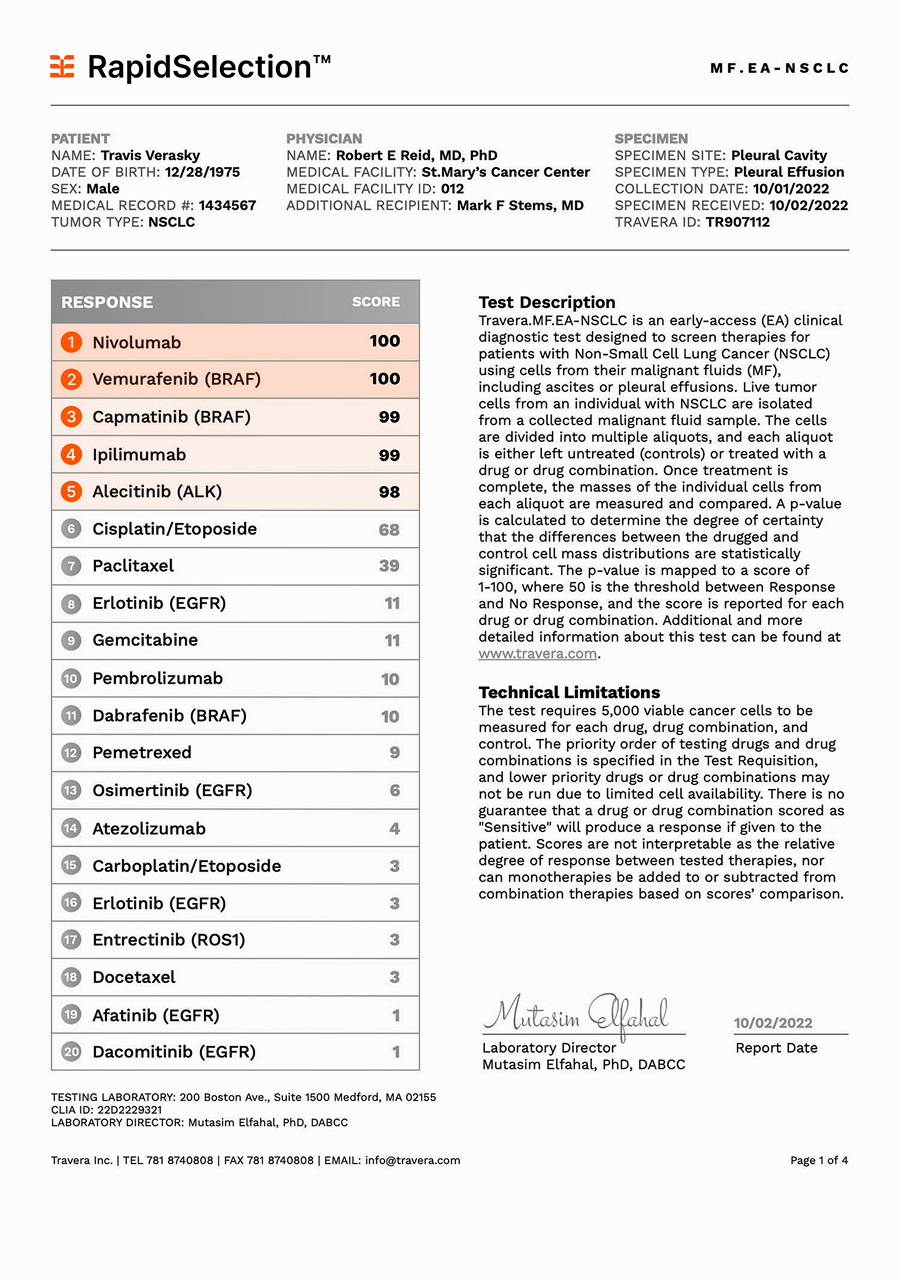
The RapidSelection™ test, you have a revolutionary tool to identify additional treatments for your cancer patients. We are moving cancer therapy beyond Standard of Care with innovative and proprietary technology developed at the Massachusetts Institute of Technology (MIT). Our test weighs individual cancer cells ex vivo with sub-picogram accuracy. Weight change begins very quickly, within a few hours of exposing the cancer cells to effective cancer drugs, and it applies to many different cancer drugs with different mechanisms of action. In an unprecedented 2-day turnaround time, you can explore alternatives for patients who have run out of options.